
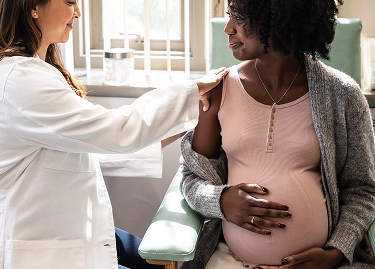
Get vaccinated while pregnant with ABRYSVO®
Today’s the day to talk to your OB-GYN about Pfizer’s ABRYSVO.
ABRYSVO is the only RSV vaccine given during pregnancy, from 32 through 36 weeks, that helps protect your baby against respiratory disease caused by RSV, from birth through 6 months.
Vaccinating while pregnant: How to pass protection from respiratory disease caused by RSV on to your newborn


ABRYSVO: a vaccine given during pregnancy to help protect newborns
Getting recommended vaccinations is an important part of prenatal care because newborns are at risk for serious respiratory illnesses from the moment they're born.
For over 20 years, pregnant women have been receiving the Tdap vaccine to help protect their babies from whooping cough.
ABRYSVO works similarly to the Tdap vaccine, helping your body create RSV antibodies that are passed to your unborn baby.
ABRYSVO should not be given to anyone with a history of severe allergic reaction (eg, anaphylaxis) to any of its components. Vaccination with ABRYSVO may not protect everyone. See Important Safety Information.
ABRYSVO was studied in a large clinical trial
Most common side effects of Pfizer’s ABRYSVO
- Pregnant women who received ABRYSVO most commonly reported pain at the injection site, headache, muscle pain, and nausea
- Low birth weight was observed in 5.1% of infants in the ABRYSVO group vs 4.3% in the placebo group
- Jaundice, also known as temporary yellowing of the eyes and skin, was seen in 7.3% of infants in the ABRYSVO group and 6.9% in the placebo group
These are not all of the possible side effects. For a full list, please see full Prescribing Information for ABRYSVO.


Get to know more about RSV in babies


Find out more about RSV and ABRYSVO


Get information delivered to your inbox

